Background
Cytokine Release Syndrome (CRS) is a well-recognized toxicity that occurs with high frequency following treatments such as T-cell engaging antibodies, Chimeric Antigen Receptor (CAR) T cells and other T-cell boosting immunotherapies. In addition to patient mortality and morbidity, the high frequency of CRS associated with these treatments represents a barrier to delivery in the outpatient setting. The need for inpatient management of CRS adds to the costs of these treatments and contributes to restricting their availability. Current treatments are dominated by reactive administration of tocilizumab, steroids or anakinra. POLB 001 is a p38 MAPK inhibitor in development for severe influenza infections and CRS. In vivo, POLB 001 acts as a broad-spectrum anti-inflammatory by potently inhibiting the expression of proinflammatory cytokines such as IL-1β, IL-6, IL-8, IL-10, TNFα, COX-2.
Methods
In this single-center, randomized, double blinded, placebo-controlled, multiple dose study (NCT05765955), 36 healthy volunteers were orally administered 30, 70 or 150 mg POLB 001 or placebo BD (3:1 active to placebo) for 7 days. Each subject was then challenged with intradermal lipopolysaccharide (LPS) and intravenous LPS which drive local and systemic inflammation, respectively. The study objectives were to study the effect of POLB 001 on inflammatory responses following LPS challenge, including cytokine analysis, exploratory analysis including PK and metabolite profile and the safety and tolerability of POLB 001.
Results
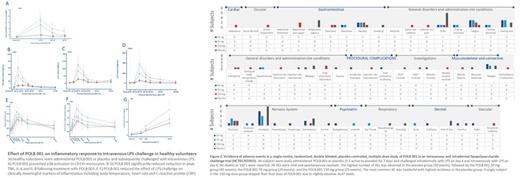
No deaths or serious adverse events occurred during the study. The most commonly reported AE was headache, with the highest number of participants and events in the placebo group (8 (88.9%) subjects and 14 events) followed by the POLB 001 30 mg group (6 (66.7%) subjects and 11 events). Other reported AEs included chills (also more frequent in placebo group) and dizziness (placebo, POLB001 30, 70, 150 mg groups, frequency 22.2%, 11.1%, 33.3%, 22.2%, respectively).
POLB 001 reduced levels of activated p38 (phospho-p38) in circulating monocytes by 32.0-60.9%. Reductions were seen in all cytokines measured. Significant reductions were seen in plasma IL-6, IL-8, IL-10 and TNFα. IL-1β levels were below the LLOQ in active and placebo groups.
LPS-driven C-Reactive Protein (CRP) was reduced by 70 and 150 mg doses of POLB 001 (33.1 and 33.3%, respectively). Intravenous LPS challenge elevated body temperature by 1°C and HR by >25 BPM with a peak 3 hours following infusion. POLB 001 significantly reduced HR elevations with an estimated difference in peak HR of -8.8 and -7.8 in the 70 and 150 mg groups respectively.
Conclusion
POLB 001 is safe and tolerable in healthy volunteers. In a human LPS challenge model driving an acute systemic inflammatory response, POLB 001 showed anti-inflammatory effects and clinically meaningful parameters of inflammation were also reduced. The results support further clinical development of POLB 001 for inflammatory diseases and CRS. POLB 001 is undergoing preclinical evaluation in humanized mouse models (NSG-(KbDb)null (IA)null) of T-cell engaging bispecific antibodies, CD19 CAR-T cell therapy for hematological malignancies and for CD28 induced cytokine storm. A Phase 2 trial of POLB 001 in Multiple Myeloma patients receiving T-cell engaging antibodies is planned.
Disclosures
Searle:Shattuck Labs: Consultancy; Abbvie: Honoraria, Other: Conference travel; Sanofi: Consultancy; Janssen: Honoraria, Other: Conference travel. Tremble:Poolbeg Pharma: Current Employment. Popat:GSK: Consultancy, Honoraria, Research Funding; Abbvie: Honoraria; BMS: Honoraria; Janssen: Honoraria; Roche: Honoraria. de Bruin:Centre for Human Drug Research: Current Employment. Moerland:Centre for Human Drug Research: Current Employment. Buckley:Poolbeg Pharma: Consultancy; Teckro: Current Employment; hVIVO Services Limited: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal